
Insights & Analysis
There’s one constant in healthcare: change. Count on us to break down the trends so you can stay up to date. Follow our take on each piece of this deep, intertwined, and often perplexing industry to find opportunities and practical approaches to move healthcare forward.

CMS Announces the Third Round of Medicare Drug Price Negotiation
CMS announced the 15 drugs in Medicare Part B and D selected for negotiation for Initial Price Applicability Year 2028.

2027 Advance Notice Materially Alters Part D Risk Adjustment
Proposed updates to the Part D risk adjustment model to further separate MA-PDs and PDPs will change plan payments for certain conditions if finalized.

FDA Programs Offer Advantages for Manufacturers in Rare Disease Space
Manufacturers can accelerate innovation and improve rare and ultra-rare disease therapy access by leveraging new and established regulatory pathways.

Driving Access to Rare Disease Care via Multi-stakeholder Collaboration: Perspectives from APAC Payers and HTA Experts
This third installment of our APAC rare disease series explores key enablers of achieving higher levels of multi-stakeholder collaboration in funding decisions for rare disease treatments.

Impact of US Drug Price Policy on Global Market Access
Given the uncertain challenge of future international price referencing by the US, Avalere Health considers implications for global market access.

How MFN Pricing in Part B May Affect Beneficiary OOP Costs
New analysis suggests the GLOBE Model would have limited impact on OOP costs, with over 99% of sampled Medicare Part B FFS beneficiaries seeing no reduction.
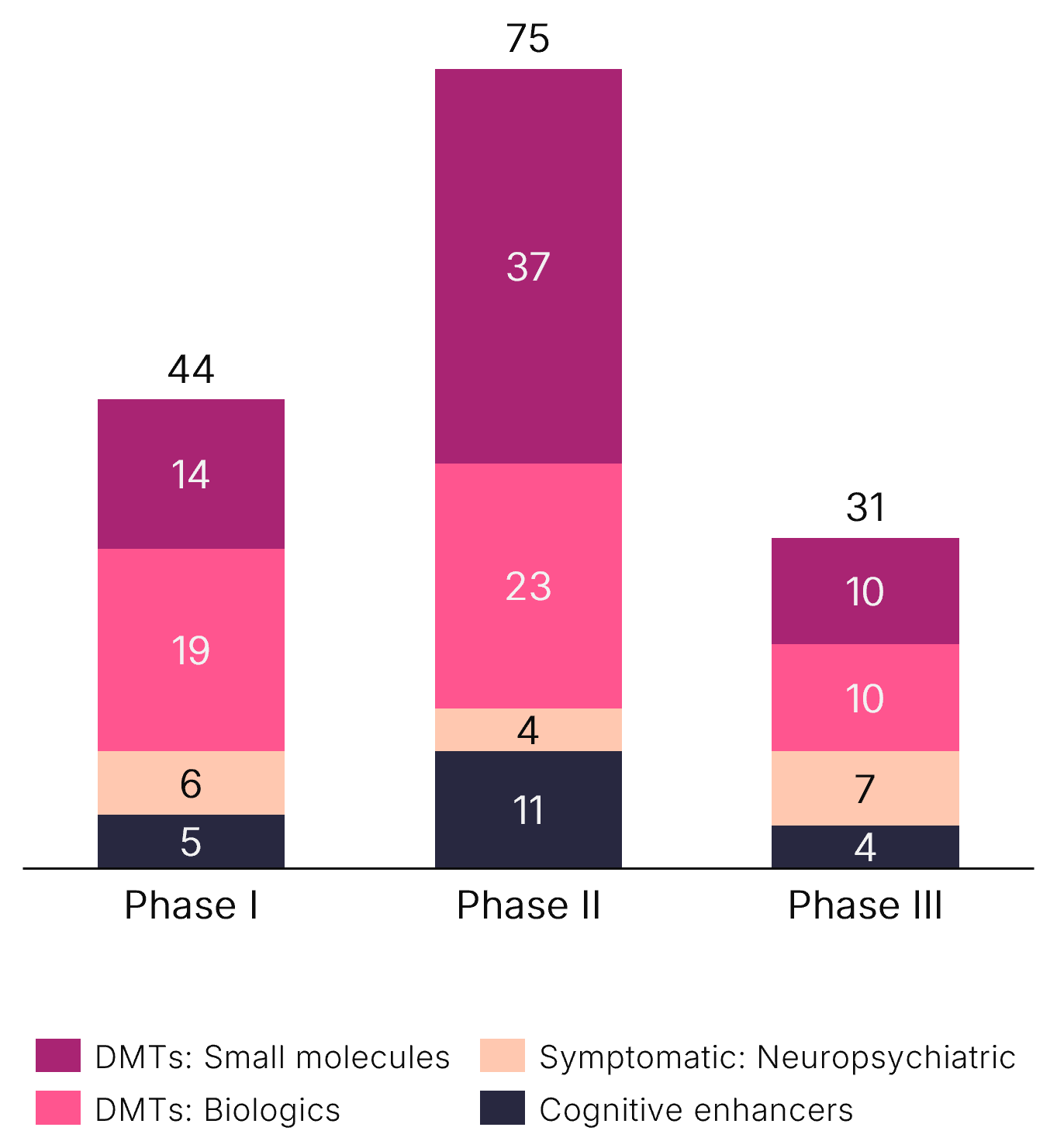
Alzheimer’s Fast-Changing Treatment Landscape and Pipeline: Need-to-Know Insights
A robust, diversified clinical development pipeline in AD requires market entrants to establish early differentiation during commercialization planning.

Now Available: 2026-2027 Coverage and Coding Regulatory Calendar
Avalere Health’s new CY 2026–2027 Coding and Regulatory Calendar supports life sciences firms’ strategic planning for critical coding deadlines, timelines, and regulatory updates.

Patients are Ready for Radioligand Therapy, But is Our Healthcare System?
Radioligand therapies show benefit across cancers, but US adoption is limited by infrastructure, reimbursement, imaging access, and care coordination gaps
Amplifying Rare Disease Patient Groups in APAC
Rare disease patient groups in APAC can amplify their impact through multi-stakeholder collaboration to achieve equitable access for people living with rare diseases.

December 2025 ACIP Insights and 2026 Emerging Priorities
The ACIP’s December meeting resulted in a key change to the pediatric immunization schedule and signaled several potential changes to US vaccine coverage and access in 2026.

340B Purchase Data Highlights Continued Program Growth
HRSA 2024 340B purchase data demonstrates continued program growth amid shifting market dynamics

White Paper: How ACIP Policies, Processes Shape the US Vaccine Landscape
A new white paper examines the historical role of the Advisory Committee on Immunization Practices and its evolution following key federal policy changes.
Bolstering Efficacy Demonstration for Cancer Drugs at HTA
New Avalere Health research explores the challenges and potential opportunities for demonstrating the value of cancer drugs to HTA agencies.
Emerging Clinical Trial Endpoints in Hemato-oncology at HTA
New research highlights the market access risks faced by manufacturers who use less traditional outcome measures to demonstrate value of innovative hemato-oncology therapies.
Environmental Impact Implementation at HTA and Beyond
Healthcare decisionmakers are increasingly considering evaluation of environmental impact; Avalere Health research explores the implications for manufacturers.

Updated Resource: State Statute Oncology Drug Coverage Report
A detailed Avalere report offers insights into state-specific statutes guiding commercial payer coverage for off-label use of oncology drugs/biologics.

CMS Recognized Greater Savings in IPAY 2027 Relative to the First Year of Negotiation
Competitive dynamics of selected drugs in 2027 drove greater savings than 2026.

PDABs Affect Nearly 8 Million Commercial and Medicaid Lives
State legislators are attempting to reduce patient costs through Prescription Drug Affordability Review Boards.

State Copay Accumulator Bans Now Affect At Least 17% of Commercial Lives
State legislators are attempting to reduce patient costs and increase access through policies that block copay accumulators and maximizers.


