A large life sciences manufacturer anticipated that some of its products may be selected for drug price negotiations under the Inflation Reduction Act (IRA). It sought to create an evidence strategy to articulate the value of its products during negotiations and thus needed to predict how CMS might weigh evidence and evaluate results. We helped the client anticipate CMS’s approach to evaluating evidence packages for products during the negotiation process, and develop strategies to strengthen those packages, to ensure that negotiated maximum fair prices (MFPs) accurately reflect the value of the client’s products.
Featured Insight
Trusted Partnership
We partner closely with clients to ensure their IRA strategy is tailored to their industry position, needs, and goals, helping clients make informed decisions and achieve long-term success.
Insight
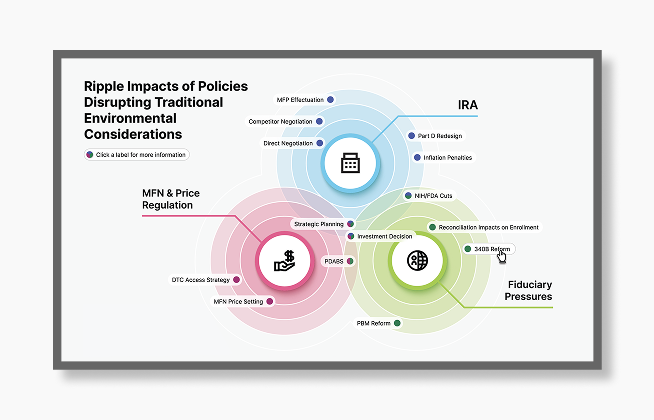
Planning for New Environmental Considerations
IRA Medicare provisions, increased focus on price regulations including Most-Favored Nation pricing, and broad fiduciary pressures have ripple effects across the healthcare ecosystem.
How Can We Help?
Engage an Expert Today.
Engage an Expert Today.


CMS Recognized Greater Savings in IPAY 2027 Relative to the First Year of Negotiation
Competitive dynamics of selected drugs in 2027 drove greater savings than 2026.

Part D Policy Change Lowers Max OOP Costs for Many Enrollees
Cost-sharing reductions from enhanced plans and EGWPs allow beneficiaries to reach the catastrophic phase with lower OOP spending than those in basic plans.

IPAY 2028 Guidance Finalizes Some Part B Details, Others Remain
The guidance clarifies some draft policies, adds MA encounter data to Part B drug selection, revises Part B 30DES calculations, and defers some issues to 2029.

White Paper: Provider Survey on Part B Negotiation Impacts
In a survey, providers say MDPNP may heighten financial pressures, raising concerns around provider sustainability and patient access

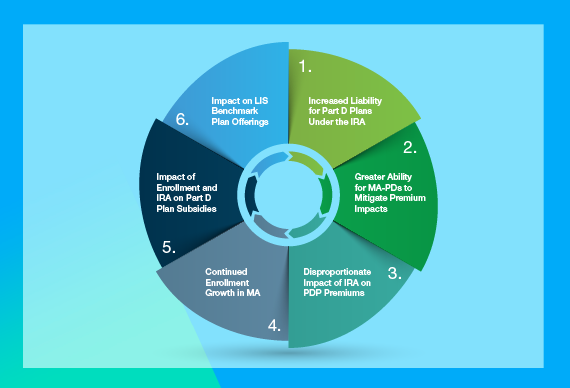
The New Stakeholder Economics of Part D After the IRA
The implementation of the IRA’s Part D redesign and Medicare drug price negotiation in 2026 will have varying impacts across plan types and therapeutic areas.

Adapting Economic Models for Medicare Negotiation
Manufacturers have several strategies at their disposal to adapt and leverage cost effectiveness evidence in preparation for Medicare drug price negotiation.

IRA, MFN, and Ongoing Fiduciary Pressures Creates Ripple Effect Across the Healthcare Ecosystem
IRA Medicare provisions, increased focus on price regulations including Most-Favored Nation pricing, and broad fiduciary pressures have ripple effects across the healthcare ecosystem.

Stakeholder Considerations for IPAY 2028 Guidance
Draft guidance for IPAY 2028 Medicare drug price negotiation includes the first-time inclusion of Part B drugs, refinement to MFP effectuation, and considerations around what qualifies as a single-source drug.
Stakeholder Considerations for MFP Effectuation in Part B
Several pathways exist to effectuate the MFP for Part B negotiation with benefits and drawbacks for stakeholders across the drug supply chain.

Early Enrollment Data Indicates More Beneficiaries Could Benefit from MPPP
Avalere Health’s analysis of early MPPP enrollment shows that only a small portion of beneficiaries who are likely to benefit from the program have enrolled.
 Please wait ...
Please wait ...