IPAY 2028 Guidance Finalizes Some Part B Details, Others Remain
Summary
The guidance clarifies some draft policies, adds MA encounter data to Part B drug selection, revises Part B 30DES calculations, and defers some issues to 2029.Background
On September 30, the Centers for Medicare & Medicaid Services (CMS) released final guidance detailing the selection and negotiation processes for Medicare Part B and Part D drugs for Initial Price Applicability Year (IPAY) 2028. This guidance builds upon the framework established in previous years and introduces several key changes from draft guidance released in May.
Key Provisions of IPAY 2028 Final Guidance
Key provisions that changed or were clarified in IPAY 2028 final guidance, relative to draft guidance, are summarized below. This list is not exhaustive, but highlights key changes for which stakeholders should be aware.
Drug Selection and Eligibility:
- Part B Drug Inclusion: For the first time, Part B drugs will be eligible for selection. To determine the selected drugs, CMS will identify the 50 highest spend Part B and D drugs separately before aggregating the lists and spending to identify the 15 drugs for IPAY 2028 negotiation.
- Medicare Advantage (MA) Encounter Data: CMS stated that it will define Part B data as inclusive of both Medicare fee-for-service (FFS) Part B claims and MA encounter data for Part B items or services. This is a change from the draft guidance, which did not include MA encounter data. Because MA encounter data does not include the plan paid amount, CMS will estimate payment based on drug units (adjusted for Medically Unlikely Edits) multiplied by the FFS payment rate. CMS will apply the appropriate FFS payment rate based on site of care and use either the published payment limit (based on average sales price) or the payments set in the Outpatient Prospective Payment System or ambulatory surgical center rates.
- Combination Drugs: In its draft guidance, CMS solicited comments on how to treat drugs with additional active ingredients that affect bioavailability but are not biologically active against the disease. CMS did not finalize any changes to its interpretation of fixed combination drug eligibility in the final guidance, but indicated that it will address this topic in IPAY 2029 rule making. Specifically, CMS noted a range of stakeholder comments that need to be considered before implementing a change and noted concern that the current policy could “create a gaming opportunity,” indicating a change is likely for IPAY 2029.
- Orphan Drug Exclusion: CMS revised guidance related to orphan drug exclusion in accordance with the Working Families Tax Cuts Act.
- Vaccines: CMS clarified that for infectious disease vaccines, it will base the definition of qualifying single source drug on the vaccines’ antigen components, effectively excluding vaccines that change formulation often from the negotiation process.
Negotiation Process:
- 30-Day Equivalent Supply for Part B Drugs: CMS finalized that for Part B claims, the number of 30-day equivalent supplies (30DES) will be calculated as the “days between service” divided by 30. Initially the draft guidance proposed a methodology equating any days less than 34 days as a full 30-day equivalent, which would not account for common real-world dosing schedules for Part B drugs on weekly or bi-weekly administration cadences. Additionally, some patients may not be adherent to labeled dosing due to clinical reasons (e.g., the patient is not healthy enough to receive an infusion on the scheduled date), which creates additional challenges related to differences in days’ supply calculations between Part B and Part D products. By adopting a methodology to divide the “days between service” by 30, CMS better captures how Part B claims are administered, although comparative challenges remain. CMS did note that it may use alternative methods when therapeutic alternatives are prescribed for a period that is meaningfully shorter than 30 days.
- Therapeutic Alternative (TA) Considerations: In its draft guidance, CMS requested comments on considering healthcare services as TAs, in addition to drugs. Based on stakeholder comments, CMS stated that for IPAY 2028, it will not consider healthcare services and clarified that it will only consider pharmaceutical therapeutic alternatives.
- Data Collection: CMS sought comment in the draft guidance on the collection of additional market data that is “forward-looking” and overlaps with the period between selection and start of the IPAY. Commenters highlighted potential risks, including data variability, increased manufacturer burden, and data confidentiality challenges; CMS did not finalize any changes and may address the issue in future rule making.
- Shorter Negotiation Period: CMS shortened the IPAY 2028 negotiation period by about two weeks and will send the final offer to manufacturers by September 30 (previously October 15).
- Clinical Consideration Town Hall: CMS will host a more robust town hall for clinical consideration for selected drugs, noting the townhall could be divided across multiple days and sessions.
- Part B Maximum Fair Price (MFP) Effectuation: In the final guidance, CMS did not finalize any topics related to Part B effectuation, including a standard default refund amount (SDRA) approach for Part B effectuation. CMS indicated that it received a broad range of comments related to Part B effectuation and will address these policies in future rule making.
- Renegotiation Criteria: CMS finalized conditions for drug renegotiation, including changes in monopoly status or significant market changes that may result in a greater than 15% change in MFP and timing of when market changes must occur.
Impact of Final Guidance on Drug Selection
The final guidance marks an important inflection point for drugs selected for negotiation. Specifically, CMS has included several key changes from the draft guidance, as well as one change possible for IPAY 2029, all with meaningful impacts.
Avalere Health maintains a dynamic negotiation forecast that projects drug selection timeline under various scenarios. The scenario options include (among other factors) the three key provisions described in the final guidance that may shift negotiation eligibility and timeline: (1) inclusions of MA encounter data in Part B drug selection; (2) exclusion of certain vaccines from negotiation eligibility; and (3) the potential to treat certain combination products as the same product in IPAY 2029. Figure 1 illustrates key changes to predicted negotiated products between IPAY 2028 and IPAY 2030 based on the three provisions noted above.
Figure 1. Anticipated Impact of Final IPAY 2028 Guidance on Drug Selection Timing
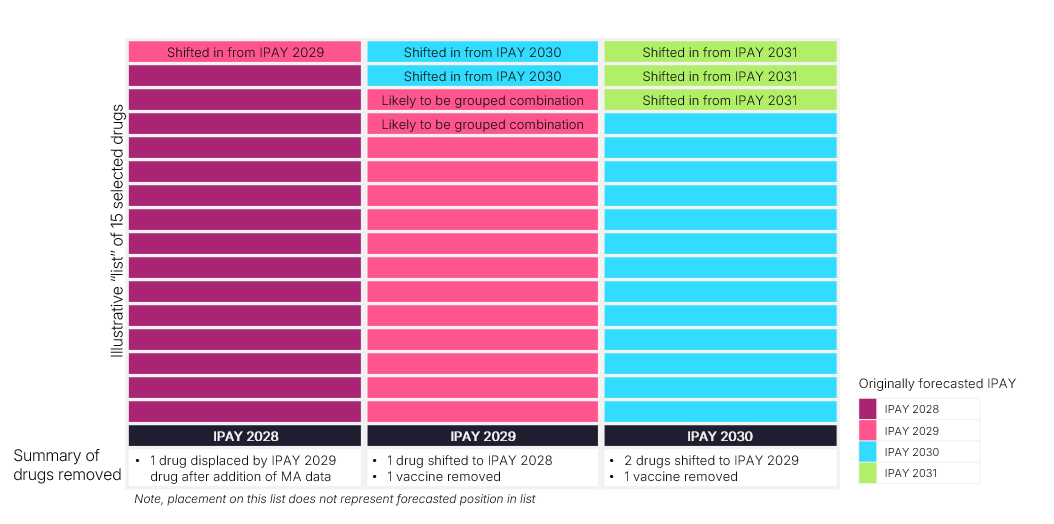
Next Steps
While CMS finalized certain aspects of it proposed guidance for IPAY 2028, there are several new considerations for stakeholders to evaluate in the future. For example, the inclusion of MA encounter data may shift timelines for selection or solidify certain product’s position on a specific IPAY list. CMS’s commentary on addressing combination products as well as Part B effectuation offer an opportunity for stakeholders to continue to engage with CMS to shape its implementation of the Medicare Drug Price Negotiation Program. Future negotiation policies will be established through rule making, which may offer stakeholders more notice of when policy changes will be announced and offer a slightly longer comment period.
Previous Avalere Health analyses have assessed the impact of MFP on provider reimbursement and potential spillover effects that can shed light on some unintended consequences of Part B negotiations. Connect with us to learn more about how Avalere Health supports client’s policy, access, pricing, contracting and channel strategy related to Part B negotiations.
Methodology
The data used in this analysis were provided by CMS through the Virtual Research Data Center (VRDC). To generate the dynamic negotiation forecast referenced above, Avalere Health used 100% Medicare Part B claims and Prescription Drug Event data accessed via the CMS VRDC for Medicare FFS beneficiaries from 2024, as well as 100% MA encounter data from 2022. Using MA encounter data, Avalere Health applied a proprietary framework to project forward and estimate 2024 MA utilization and payment. The approach Avalere used to estimate MA spending aligns with the framework described in the IPAY 2028 final guidance.
Avalere Health developed the dynamic list framework in accordance with CMS guidance on product eligibility, negotiation timelines, and total expenditure calculations, and leveraged supplemental sources to inform assumptions and eligibility criteria.
Avalere Health compared 2022 utilization between Medicare FFS Part B claims and MA encounter data to determine variation in product use between groups. Avalere Health also assessed total drug spending in calendar year 2024 for beneficiaries identified with COVID-19 based on diagnosis or medication use and found that this cohort accounted for 10.4% of total drug spend ($15.7 billion).







