What Would a USPSTF Overhaul Mean for Preventive Care Access?
Summary
Reported plans to reshape the USPSTF align with recent changes to ACIP and may shape the future of preventive care recommendations and access.Recent reporting from the Wall Street Journal indicates that US Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. is planning to take the unprecedented step of dismissing all current members of the US Preventive Services Task Force (USPSTF). This reporting follows the Supreme Court’s decision in Kennedy s. Braidwood affirming the HHS Secretary’s authority over the Task Force and its membership.
The Wall Street Journal story sparked immediate outcry across the medical and public health communities, including in a letter from the American Medical Association urging Sec. Kennedy to retain the current USPSTF membership and uphold the integrity of the Task Force’s evidence-based process. Additionally, over 100 health organizations wrote to Congress asking that it “protect the integrity of USPSTF from intentional and unintentional political interference” following the Braidwood decision. The report also drew direct attention from Congress; Senators Angus King (I-ME) and Elizabeth Warren (D-MA) introduced a resolution to affirm support for the USPSTF.
USPSTF is an independent panel of 16 volunteer experts in preventive services issues evidence-based recommendations on these services. Recommendations with an “A” or “B” grade must be covered without cost sharing per the Affordable Care Act (ACA). Its recommendations inform access to cancer screenings, counseling services, and preventive medications like HIV pre-exposure prophylaxis.
Changes to Another HHS Advisory Panel
Sec. Kennedy’s planned overhaul of the USPSTF mirrors the recent reconstitution of the Advisory Committee on Immunization Practices (ACIP); all existing members were dismissed and replaced with new advisors more closely aligned with the Secretary’s agenda. These members’ documented positions on vaccines are informing proposed changes to the childhood vaccination schedule and recently led to a recommendation against the use of influenza vaccines containing thimerosal. Despite scientific evidence finding no risk or harm associated with the use of thimerosal, Sec. Kennedy accepted this recommendation in July 2025.
Potential Impacts and Where We Go from Here
The reported forthcoming dismissal of USPSTF members may be rooted in the administration’s broader agenda. Wall Street Journal sources cite the Task Force’s clinical guidance and recommendations related to health disparities, its use of inclusive language (e.g., “pregnant persons”), and its acknowledgment of structural racism as potential justification for this latest shift. Going forward, this ideological stance may influence how public health policy is created and perceived by the administration, potentially threatening the credibility of federal advisory committee decisions.
The USPSTF has historically relied on robust, evidence-based processes and been perceived as independent from external influence and political pressures. Potential replacement of its membership, particularly if undertaken outside of the established appointment process, could undermine this structure and create uncertainty in federal guidance and coverage mandates. For example, payers may face destabilized benefit design, providers may see shifting preventive care expectations; and patients across a broad demographic and geographic landscape may face limited access to essential, no-cost preventive services.
Table 1 assesses the potential impact a complete replacement of current USPSTF members may have on select Grade “A” and “B” recommendations, informed by internal subject matter expertise and public statements from the current Trump administration. The impact potential ratings consider public health significance, population reach, equity implications, likelihood of political scrutiny, and dependence on preventive coverage protections (e.g., ACA).
Table 1: Potential Impact of USPSTF Overhaul on Grade A/B Recommendations
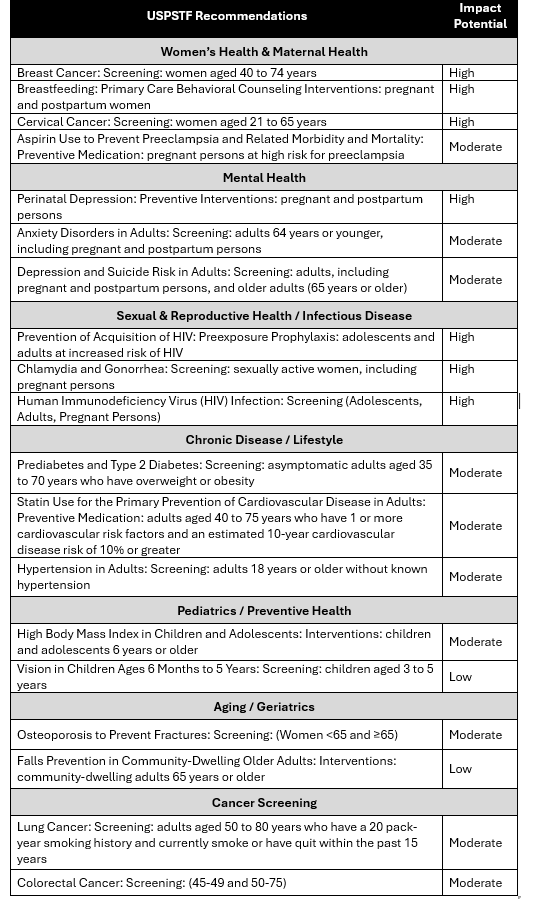
Impact Potential reflects an internal assessment of how disruptive changes to each USPSTF recommendation may be if the Task Force is overhauled. Ratings consider public health significance, population reach, equity implications, likelihood of political scrutiny, and dependence on preventive coverage protections (e.g., ACA).. High = broad disruption risk; Moderate = targeted or conditional impact; Low = minimal near-term consequence.
Looking Ahead
As political dynamics reshape federal advisory panels, Avalere Health can help manufacturers, plans, providers, and patient groups protect patient access through strategies rooted in evidence-based science, and shape preventive care policy now and for the future. With deep expertise across evidence generation, regulatory strategy, public policy, and quality frameworks, we offer a cross-functional approach tailored to the nuances of preventive care. Our team has a proven, successful track record supporting diagnostic, biopharmaceutical, and digital health companies engaging the USPSTF to unlock access and drive adoption. To learn more about how Avalere Health can assist you, connect with us.






