Medicare Draft Coverage for Alzheimer’s Drugs May Challenge Access
Summary
Thirty-one percent of rural Medicare fee-for-service beneficiaries with early-onset Alzheimer’s disease or a mild cognitive impairment diagnosis do not have access to a hospital outpatient department in their county, and fewer than 1% live near an Alzheimer’s disease research center.On January 11, the Centers for Medicare & Medicaid Services (CMS) issued a draft national coverage determination (NCD) decision memo that outlines how Medicare may cover aducanumab (Aduhelm™) and future Food & Drug Administration-approved monoclonal antibodies (mAbs) directed against amyloid plaque for the treatment of Alzheimer’s disease (AD). The CMS intends to implement a Coverage with Evidence Development (CED) component to the NCD, which provides coverage to this class of drugs only within the context of CMS-approved or National Institutes of Health (NIH)-supported randomized controlled trials. The CMS also proposes to limit eligible clinical trials to the hospital outpatient department (HOPD) setting.
To examine the potential impact of the draft NCD decision memo, Avalere conducted an analysis to understand beneficiary access to AD mAbs through randomized controlled trials if the CMS maintains the coverage requirements outlined in the draft NCD decision memo.
Medicare Beneficiary Access to HOPD
The draft NCD decision memo states that all trials must be conducted only in the HOPD setting. This requirement could limit access for beneficiaries who do not have easy access to a HOPD and pose challenges for caregivers who need to transport patients to CED-eligible sites. Avalere’s analysis found that among all Medicare FFS beneficiaries with early-onset AD or mild cognitive impairment, 7% do not live in a county with a hospital outpatient department. Among rural beneficiaries with these diagnoses, 69% have access to a HOPD in their county of residence.
Access to AD Academic and Research Centers
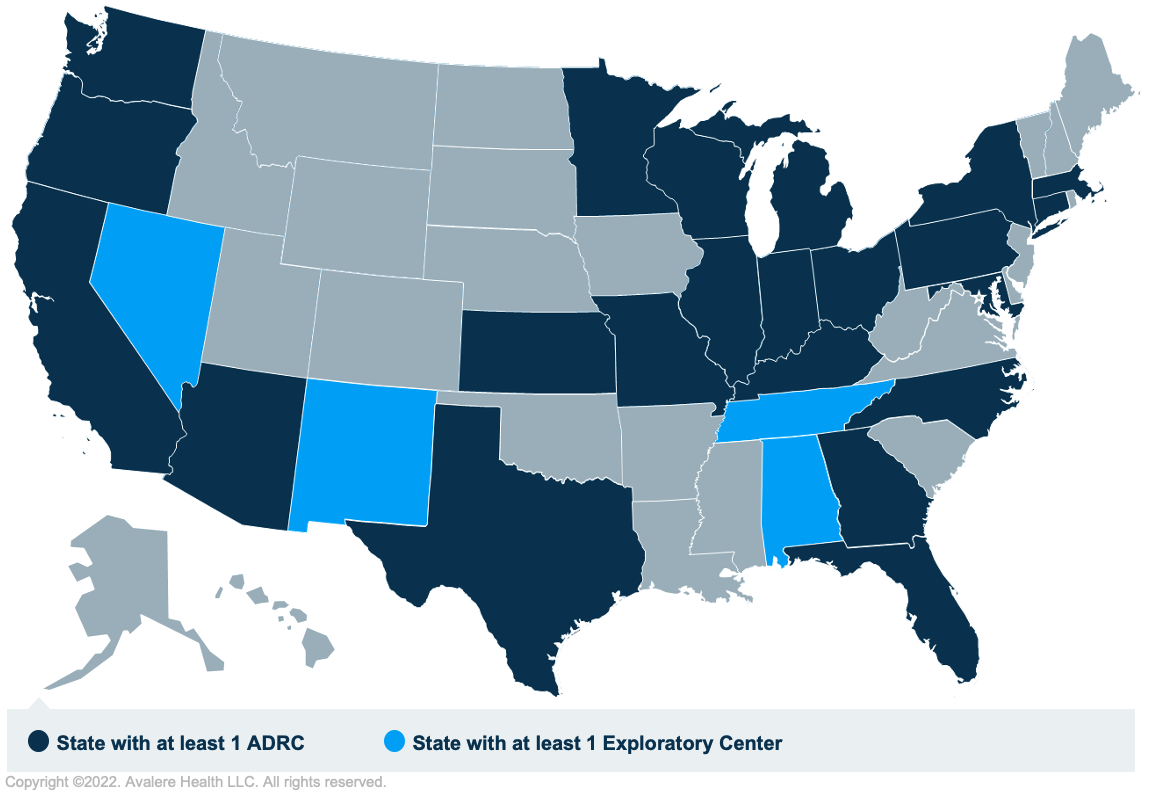
While the draft NCD decision memo allows coverage for the novel AD mAbs within qualified clinical trials in the HOPD setting, it remains unclear how many hospitals will be willing and able to conduct the clinical trials required by the CMS. Research centers that will conduct randomized controlled trials for the CED requirement will likely include AD research centers (ADRCs), which are NIH centers of excellence at major medical institutions across the US that conduct AD clinical trials and provide infrastructure to facilitate the Alzheimer’s Clinical Trials Consortium.
ADRCs already host trials sponsored by the manufacturers of mAbs directed against amyloid plaque for the treatment of AD. But ADRCs also tend to be highly concentrated in certain parts of the country (Figure 1), which may lead to access barriers for Medicare beneficiaries eligible for AD mAb treatment. Currently there are 33 ADRCs and 4 exploratory ADRCs nationwide.
Avalere’s analysis found that among all Medicare fee-for-service (FFS) beneficiaries with early-onset AD or mild cognitive impairment, 21% have an ADRC in their county of residence and 79% do not. But among rural beneficiaries with the same diagnoses, fewer than 1% have an ADRC in their county.
Source: National Institute on Aging, “Alzheimer’s Disease Research Centers,” September 2021.
The CMS collected stakeholder comments through February 10 and aims to issue the final NCD decision memo by April 11. Until the final coverage memo is released, coverage decisions for mAbs directed against amyloid for the treatment of AD will continue to be determined by local Medicare administrative contractors on a case-by-case basis.
To stay up to date with changes in Medicare, connect with us.
Methodology
Under a research-focused data use agreement with the CMS, Avalere examined data from the Medicare Part B FFS claims to identify beneficiaries with an AD or dementia diagnosis code. Avalere identified beneficiaries in 2020 with at least 1 of the following diagnosis codes: “G30.0: Alzheimer’s disease with early onset” and “G31.84: Mild cognitive impairment, so stated.”
Avalere used beneficiary ZIP codes available in the Master Beneficiary Summary File to limit the sample of beneficiaries with AD or dementia to those living in rural areas based on the Rural-Urban Commuting Area (RUCA) codes. We classified rural ZIP codes as RUCA codes 7–10. The locations of HOPDs were identified using the CMS list of Medicare outpatient hospital providers, and the locations of ADRCs were identified using the NIH list of center locations. HOPD and ADRC locations were then mapped to counties The proportion of beneficiaries in rural ZIP codes that have a HOPD or ADRC in their county was then determined dividing the number of beneficiaries in a rural ZIP code with an HOPD or ADRC within the county by the number of beneficiaries residing in a rural ZIP code.




