Vaccine Policy and Access Under New HHS Leadership
Summary
Policy actions in the first days of the Trump administration raise questions about the future role of key vaccine advisory committees.During its early days in office, the Trump administration issued a directive pausing health agencies’ external communications, including Federal Register notices, news releases, and other key publications. This move was likely made to allow time for the appointment of new directors and agency leaders; communication is expected to resume February 1.
Some key agency appointments have already been rolled out, but stakeholders are also watching closely the pending confirmation hearings for Robert F. Kennedy, Jr., the nominee for HHS Secretary, who will address the Finance Committee on January 29 and the Health, Education, Labor and Pensions Committee on January 30. If confirmed, Kennedy may elevate new public health and immunization policy priorities, including those related to vaccine approvals, safety, and coverage. Stakeholders should monitor the confirmation hearings and prepare for any potential implications for vaccine access.
Short-Term Considerations
Given the communication freeze in the lead up to the Kennedy confirmation process, previously scheduled 2025 vaccine advisory committee meetings have been postponed. For example, the National Vaccine Advisory Committee (NVAC) was scheduled to discuss priorities for the 2026–2030 National Vaccine Strategic Plan at the end of February and the Advisory Commission on Childhood Vaccines (ACCV) was scheduled to discuss the Vaccine Injury Compensation Program (VICP) this week. These meetings were postponed without public notice and rescheduling plans remain unclear.
In addition, the Centers for Disease Control and Prevention (CDC) did not publish its Morbidity and Mortality Weekly Report, which serves as a key resource for monitoring infectious disease and public health trends.
Historically, federal advisory committee activities and CDC publications have proceeded as planned amid administrative changes, so these changes and pauses underscore heightened uncertainty for vaccine policy. Should these delays persist for a longer period, it could delay critical vaccine use recommendations and create challenges to public health implementation at all levels, including federal and state programs and immunization jurisdictions.
Figure 1. Scope and Oversight of Key Vaccine Advisory Committees*
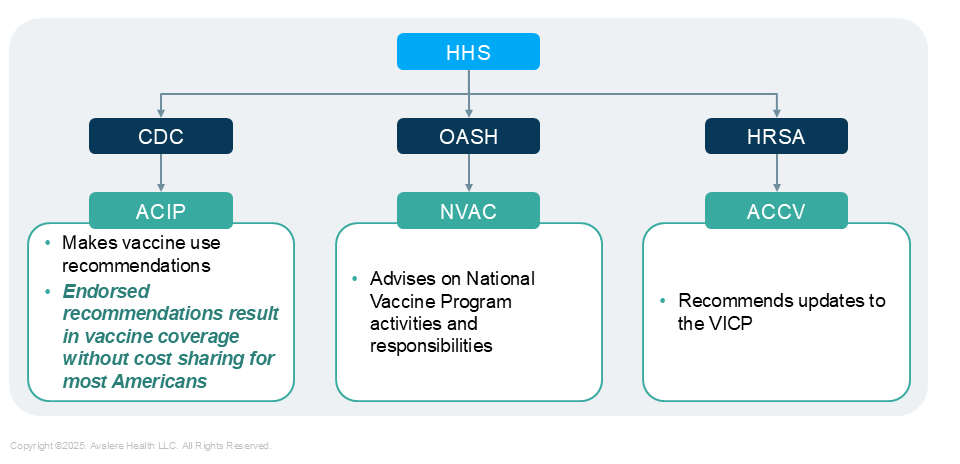
*Not all-inclusive. HHS: US Department of Health and Human Services; HRSA: Health Resources and Services Administration; OASH: Office of the Assistant Secretary for Health
When external communication resumes, certain administration agency officials could be in place to facilitate communication as other leadership positions await Senate confirmation (including the Food and Drug Administration Commissioner and, for the first time, the CDC Director). Selection and confirmation of these individuals will ultimately determine who sets the priorities for the top US public health agencies, potentially underscoring new expectations and oversight for healthcare policymaking.
Longer Term Considerations Under New HHS Leadership
- While the ACIP considers clinical safety evidence in its decision making, Kennedy’s stated prioritization of vaccine safety could lead to increased scrutiny of the Committee’s recommendation development process. ACIP recommendations require endorsement by the CDC Director, an executive function delegated by the HHS Secretary, before they can inform coverage policy.
- ACCV may also play a larger role in vaccine policy and communications related to safety. Its charter allows the Commission to recommend changes to the Vaccine Injury Table, recommend vaccine injury-related research activities, and advise on developing or revising vaccine information materials. These materials include Vaccine Information Statements (VIS), which are published by the CDC and required to be given to patients before administering a vaccine.
- Kennedy may also prioritize VICP reform. Rep. Lloyd Doggett (D-TX-37) introduced the VICP Modernization Act in the previous Congress, which would increase the number of vaccine injury claim adjudicators, increase the statute of limitations for filing claims, and add adult vaccinations to the Injury Table. A nonprofit organization started by Kennedy, however, supported a different bill that would remove liability protections for manufacturers of COVID-19 vaccines, increasing their financial responsibility for any COVID-19 vaccine-related injury claims.
What’s Next
While Kennedy’s potential confirmation and the days following could provide clarity on what to expect from future health agency communications, much remains uncertain. With that in mind, new agency leadership and policy changes require stakeholders to remain adaptable. To learn more about how Avalere’s Vaccines Team can help you prepare flexible evidence, policy, and access strategies in the face of uncertainty, connect with us.
Appendix: Advisory Committee Authorizing Statute
| Organization | Recommendation |
|---|---|
| ACCV | • 42 USC §300aa–19(a): Established ACCV • Federal Advisory Committee Act (FACA), 5 USC Chapter 10: Governs ACCV |
| ACIP | • 42 U.S.C. §2l7a: Established ACIP • Federal Advisory Committee Act (FACA), 5 USC Chapter 10: Governs ACIP • 42 U.S.C. §1396s(c)(2)(B)(i) and 42 U.S.C. §1396s(e): Establishes ACIP’s role in the Vaccines for Children program • 42 U.S.C. §300gg-13(a)(2): Links ACIP recommendations to insurance coverage requirements for vaccines |
| NVAC | • 42 USC §300aa–5(a): Established NVAC • Federal Advisory Committee Act (FACA), 5 USC Chapter 10: Governs NVAC |




