Newborn Screening: Landscape and Rare Disease Developments
Summary
Novel approaches to newborn screening of rare diseases could shape the future of federal and state screening guidelines.Newborn screening (NBS) is a public health program that helps identify rare conditions that may affect a child’s long-term health or survival. The overarching goal of this federal program is to allow early treatment, leading to reduction or elimination of the disease symptoms and its downstream impacts. About 4 million babies born in the United States are screened at birth each year. Through NBS, approximately 13,000 children are identified annually with a congenital condition (condition that is present at birth) such as a rare metabolic, endocrine, hemoglobin, and “other” (hearing and congenital heart disease) disorder.
All states are required to operate an NBS program. State public health programs are encouraged to screen for disorders included in the national Recommended Uniform Screening Panel (RUSP), but it is up to individual states to determine which conditions will be included on their screening panels.
Recommended Uniform Screening Panel
The federal Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) issues the RUSP and advises the Health and Human Services (HHS) Secretary on the most appropriate application of the NBS tests, technologies, policies, and guidelines.
The RUSP is a list of disorders that the Secretary recommends for states to screen as part of their state universal NBS programs. The panel includes 38 primary and 26 secondary rare conditions that can be detected either through laboratory screening of dried blood spots or point-of-care screening. According to Health Resources and Services Administration (HRSA), “non-grandfathered health plans are required to cover screenings included in the HRSA-supported comprehensive guidelines without charging a co-payment, co-insurance, or deductible for plan years beginning on or after the date that is one year from the Secretary’s adoption of the condition for screening.”
Adding a condition to the RUSP is a multistep process that may take more than one year to complete. To add a condition to the RUSP, requestors must submit a comprehensive evidence package. The committee will then review and hold a preliminary vote on the recommendation, followed by external expert review of the evidence, after which the committee will review again and vote on the final recommendation to be adopted by the HHS Secretary.
Stakeholders interested in adding a rare disease to the NBS panel have two potential avenues: request a condition to be added to the RUSP at the federal level or engage with individual states to have a disease added to the state NBS program.
Table 1: Characteristics of NBS Programs
| Federal RUSP | State NBS Program | |
|---|---|---|
| Scope | National | State |
| Enforcement | Recommended | Regulated |
| Number of Conditions | 38 primary and 26 secondary conditions | Between 33 and 75 |
| Funding | HRSA | State-determined fees; health insurance; Medicaid/Children’s Health Insurance Program |
| Time to Add a New Condition | From 21 months to 10 years | Several months to several years |
| Level of Engagement to Add a New Condition | High | Varies by State |
RUSP Alignment Legislation
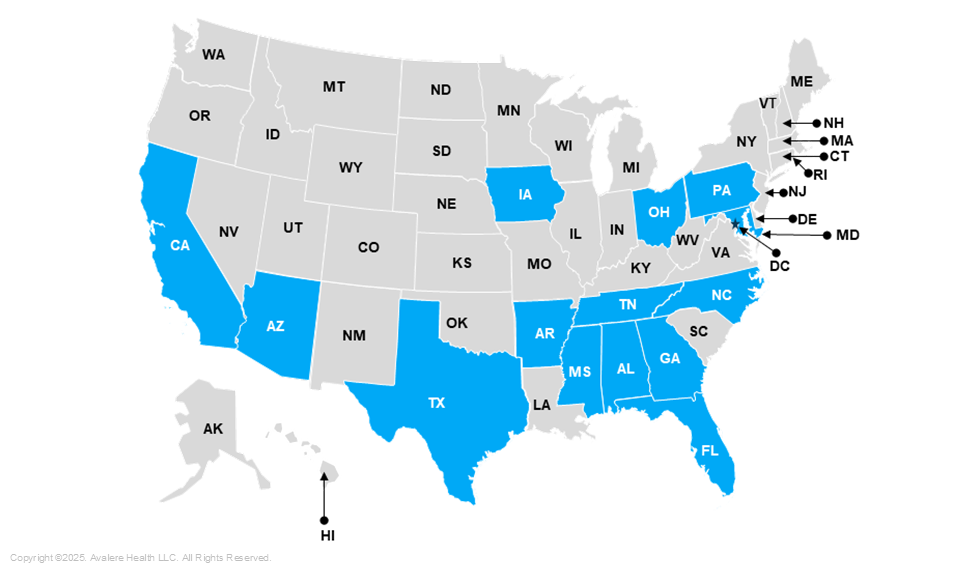
Several states’ have laws that align their NBS program with RUSP, meaning that their state will screen newborns for any condition on the RUSP, implement a timeline for including a condition to the state panel, and ensure appropriate resource allocation to meet the recommendations. These laws expedite the process of adding disorders included on the RUSP to state panels once they are approved by the ACHDNC. There are currently 14 states that have enacted RUSP alignment legislation, with Tennessee and Alabama being the most recent ones (Figure 1).
Figure 1: States with RUSP-Aligned Newborn Screening Laws
Novel Approaches to Newborn Screening
It takes an average of 4.8 years for a patient with a rare or ultra-rare condition to receive an accurate diagnosis, and they may have to see more than seven specialists during this process. Of more than 10,000 known rare diseases, RUSP includes only 64.
Many researchers recognize the need to reduce the duration of patients’ diagnostic odyssey through implementation of novel approaches to NBS, such as rapid genomic sequencing. Multiple studies are evaluating the impact of adding genomic sequencing to NBS. In the United States, these studies include BeginNGS, BabySeq, and Early Check. Ex-US programs include 100,000 Genomes Project in the United Kingdom, Initiative on Rare and Undiagnosed Diseases in Japan, Genomics Health Futures Mission in Australia, and the Saudi Human Genome Program in Saudi Arabia.
There are several implications of genomic sequencing, including ethical considerations (e.g., privacy, types of results to be returned to parents, psychological impacts of knowing), impact on the overall healthcare system, implementation, governance, and social determinants of health. The initial results of the multiple studies indicate that some of these impacts may not be realized if genomic sequencing is implemented on a larger scale. Although further studies are required, the initial results are very promising.
Deeper Dive
Avalere applies our expertise in state policy assessment and evidence generation planning to help stakeholders meet their business objectives through effective commercialization. To learn more about addressing unmet needs in rare disease and develop federal or state-specific engagement NBS strategies, connect with us.






