New CAR-T Policies Affect Access, Reimbursement
Summary
Medicare CAR-T payment will increase in FY 2026, improving hospital reimbursement; CMS will consider shifting to market-based rate-setting in future rulemaking.In the fiscal year (FY) 2026 Inpatient Prospective Payment System (IPPS) rule, the Centers for Medicare & Medicaid Services (CMS) finalized several policies that would impact provider reimbursement for chimeric antigen receptor T-cell (CAR-T) and other cell and gene therapies (CGTs). Per the rule, CMS will continue to use its Medicare Severity Diagnosis-Related Group (MS-DRG) for CAR-T treatment stays, but with a 17% increase in the base rate. The rule also lowers reimbursement rates for CAR-T therapies provided as part of a clinical trial. The financial impacts of these provisions will vary by hospitals, with some providers’ costs continuing to eclipse the increased base payments.
Background
Since the first Food & Drug Administration (FDA) approval of a CAR-T product in 2017, stakeholder concerns have persisted over how the Medicare program would reimburse for these products. CAR-T therapies are commonly administered in the inpatient setting and have a significant cost for providers (e.g., average sales prices [ASPs] exceeding $450,000). Hospital inpatient reimbursement is calculated on a case-by-case basis using an MS-DRG base payment rate that is adjusted for factors such as hospital geography, diagnosis, case severity, and discharge status. Additional reimbursement can be provided through new technology add-on payments (NTAPs) and outlier payments.
For FY 2025, inpatient stays with CAR-T treatment are currently assigned to MS-DRG 018, which has a base reimbursement rate of $269,139. Outlier payments are available to hospitals to cover extremely costly cases when the costs exceed the total of the MS-DRG payment, the NTAP amount (if applicable), and the current fixed-loss threshold of $46,147. Even with these adjustments, Medicare reimbursement for CAR-T cases today sometimes fails to cover total hospital costs, which can negatively impact provider uptake and patient access.
Payment Changes for CAR-T Cases
As a result of an increase to the base operating and capital rates for all IPPS payments and an increase in the proposed relative weight for MS-DRG 018, the proposed base payment for CAR-T cases in FY 2026 will increase by 16.8%, to $314,231. This final rate is slightly higher than the rate in the proposed rule.
High-Cost Outlier Payments
The finalized fixed-loss threshold for FY 2026 is $40,397, a 13% decrease over the current threshold and lower than the proposed threshold of $44,305. This decrease is a departure from the steady increases of recent years; between FYs 2020 and 2025, the outlier threshold increased by 74%. For CAR-T cases, which are more likely than other inpatient stays to qualify for outlier payments, fluctuations in the threshold are particularly important, as they dictate the losses hospitals must incur before qualifying for an outlier payment. The combination of an increased base rate for CAR-T cases and a lower outlier threshold would increase provider reimbursement (see Figure 1).
Adjustment for Clinical Trial Cases
CMS reimburses CAR-T clinical trial cases, which do not incur drug costs, at a lower rate than non-clinical trial cases. Using its standard approach, CMS finalized an adjustment factor of 0.16 to the relative weight of MS-DRG 018 for a base rate of $50,277. This is a 43% decrease over the current clinical trial payment rate (using an adjuster of 0.33). The finalized clinical trial adjuster and rate are also lower than the proposed rule adjustment factor of 0.23 (which would have produced a $72,260 base rate). Therefore, hospitals will face lower payments for CAR-T cases when provided as part of a clinical trial at the beginning of the new fiscal year.
Product NTAP Assessments
CMS evaluated several CGT products for NTAP in FY 2026 and considered public comments on whether each product meets newness, cost, and clinical improvement criteria required for NTAP status.
NTAP was approved for two cell therapies:
- Liso-cel (BREYANZI®), a CAR-T treating chronic lymphocytic leukemia or small lymphocytic lymphoma. The product was previously approved for NTAP in FY 2021-2022 for large B-cell lymphoma and will have a maximum NTAP payment of $316,860
- Afamitresgene autoleucel (TECELRA®), an autologous T-cell receptor therapy for the treatment of advanced synovial sarcoma, will have a maximum NTAP payment of $472,550
NTAP was denied for one CAR-T therapy: Obe-cel (AUCATZYL®), which is indicated for the treatment of acute lymphoblastic leukemia. It was deemed “substantially similar” to TECARTUS® and therefore failed to meet the newness criterion.
Figure 1. Hospital Reimbursement Example for CAR-T Cases Under IPPS: Final FY 2025 vs. Proposed FY 2026 vs. Final FY 2026
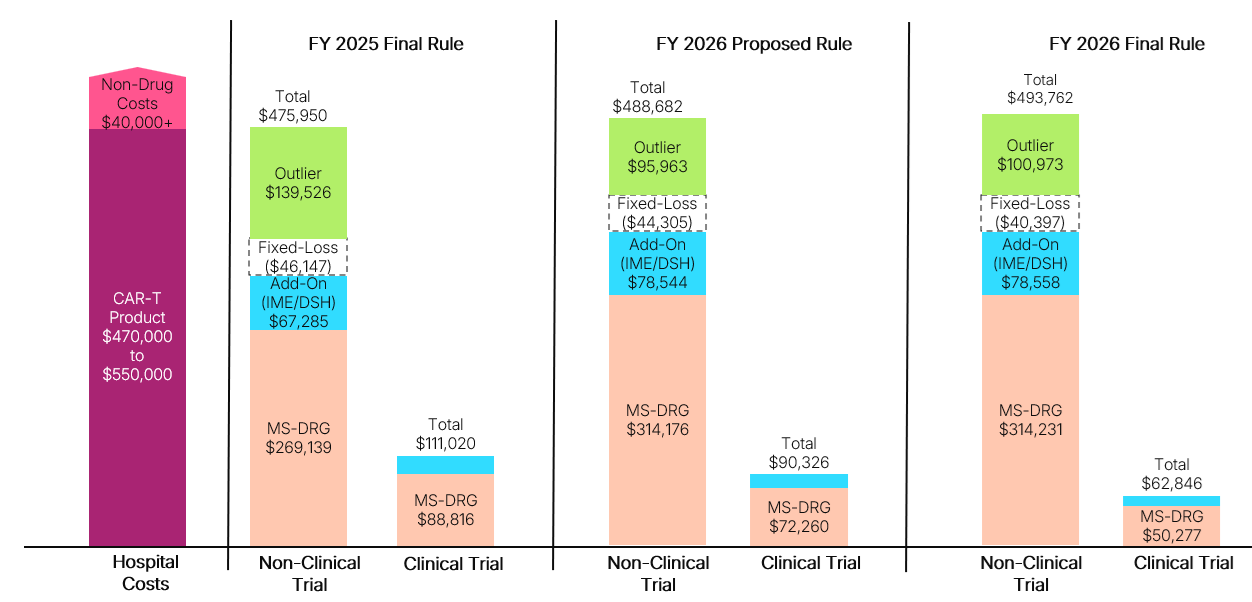 DSH: Disproportionate Share Hospital; IME: Indirect Medical Education
DSH: Disproportionate Share Hospital; IME: Indirect Medical Education
Assumptions: Hospital charges for CAR-T episode are kept constant across all examples, consistent with the geometric mean charges included in the FY 2025 Proposed Rule after outliers removed (AOR) file ($1,864,674); Hospital has an average operating and capital cost-to-charge ratio of 0.3, which influences CMS calculations of hospital costs;; Hospital receives add-on payments that stem from payments for IME and DSH adjustments, which are calculated as a percentage of the base MS-DRG. In this figure, there is an assumed IME factor of 0.2 and DSH adjustment of 0.05; Hospital area wage index is 1.0, meaning no adjustment is made to the labor-related share of the standardized amount.; Hospital received outlier payment, which is only made once hospital losses exceed the fixed-loss amount. Outlier payments provide 80% of excess costs beyond this threshold.
Key Considerations
Stakeholders should consider several implications stemming from finalized FY 2026 changes for existing assets and for future CGTs.
- Stability of MS-DRG 018: Total reimbursement will vary by hospital and case, but the increase in reimbursement for FY 2026 will generally support improved cost recovery for providers. However, profitability by case will become increasingly variable given the continuing assignment of therapies to the MS-DRG. Specifically, additional immunotherapies mapped to the MS-DRG can lead to fluctuations in the base rate and could eventually lead CMS to consider splitting the MS-DRG depending on the number of cases and differences in resource costs.
- NTAP Eligibility: As the number of CAR-T products available grows, it may be increasingly difficult for new products to clearly satisfy criteria for newness, cost, and clinical improvement relative to on-market products. For example, obe-cel was denied NTAP due to substantial similarity to a product that previously had an NTAP. However, the approval of Breyanzi’s NTAP application for a new indication could be a positive signal for CAR-T manufacturers studying use and seeking approval in new indications.
- Market-Based MS-DRG Payment Rates: The calendar year 2026 Outpatient Prospective Payment System (OPPS) proposed rule includes a proposal that could significantly impact IPPS payment rates. CMS proposes to use median Medicare Advantage negotiated rates by MS-DRG to set the rates for IPPS fee-for-service (FFS) cases starting in FY 2029. This could be particularly impactful for CAR-T, where negotiated rates for MS-DRG 018 may differ from the current base rates available under the FFS methodology. CMS describes this proposal as an effort to align MS-DRG relative weights with market-based dynamics rather than relying heavily on hospital charges. The final OPPS rule will be released in November 2025.
- Future of Inpatient Treatment: Differences in Medicare reimbursement methodology for the inpatient versus outpatient setting can often result in higher reimbursement for CAR-T in the outpatient setting, where CAR-Ts are typically separately paid at ASP+6%. While most cases are currently provided in the inpatient setting, the recent removal of Risk Evaluation and Mitigation Strategy requirements for CAR-T products could speed adoption of outpatient administration or administration in community-based, non-hospital settings. Additionally, the revised safety guidelines could potentially support shorter lengths of inpatient stays, influencing hospital costs/economics for the CAR-T journey. An Avalere Health analysis showed that the average length of stay for Medicare FFS beneficiaries is 24 days, which may progressively decrease based on the revised safety guidelines for the CAR-T products and reduce pressure on patient/caregiver ancillary costs of treatment.
- Changes in Line of Treatment and Setting of Care: As CAR-T products are approved for earlier lines of treatment, providers and payers will increasingly have to evaluate the benefits of earlier intervention with a CAR-T product, weighing reimbursement, safety, and patient preference. Community expansion of CAR-T could significantly change access dynamics for patients; in an assessment of triple-class exposed multiple myeloma patients who may be eligible for CAR-T treatment, Avalere Health found that only 18% live in ZIP codes that are generally served by authorized treatment sites. In contrast, Avalere Health also found that 58% of triple-class exposed multiple myeloma patients are within a 30-minute drive of a treatment site that administers bispecific products. Broadening the CAR-T treatment center footprint could allow more patients to receive treatment, as eligible and appropriate.
Next Steps
Carefully monitoring reimbursement for these innovative products will allow CGT manufacturers, providers, and payers to engage other stakeholders based on anticipated developments. To discuss how Avalere Health can support your business on issues related to commercialization, NTAP proposal submissions, provider reimbursement, or policy developments, connect with us.




